Zepbound® Side Effects: Common Symptoms and How to Manage Them


Zepbound® side effects explained—what’s common (nausea, diarrhea, constipation) and what helps, plus warning signs that mean you should call your provider.
- The most common Zepbound® side effects involve your stomach and digestion, such as nausea, diarrhea, constipation, and vomiting.
- Side effects may appear with dose increases, but people often notice that they get better over time.
- There are some rare but serious risks, like pancreatitis, gallbladder issues, kidney problems from dehydration, severe allergic reactions, and low blood sugar. These are more likely if you also use insulin or a sulfonylurea.
- Let your healthcare team know that you take Zepbound® before you have any procedures or surgery. GLP-1 medications can slow gastric (stomach) emptying, and rare aspiration events have been reported (especially with anesthesia or deep sedation).

The Basics
If you’re searching for Zepbound® side effects, you’re probably curious about two things:
- How will I feel on this medication?
- If I have side effects, what can help, and how do I know if it’s something serious?
Zepbound® is the brand name for tirzepatide, a prescription injection you take once a week. It’s used with lifestyle changes for chronic weight management (it’s also approved for moderate to severe obstructive sleep apnea in adults with obesity).
In this guide, we’ll go over the most common side effects, what can help, and the warning signs that mean you should contact your healthcare provider right away. This is for general education purposes, not medical advice, so always follow your prescriber’s instructions.
The Most Common Zepbound® Side Effects
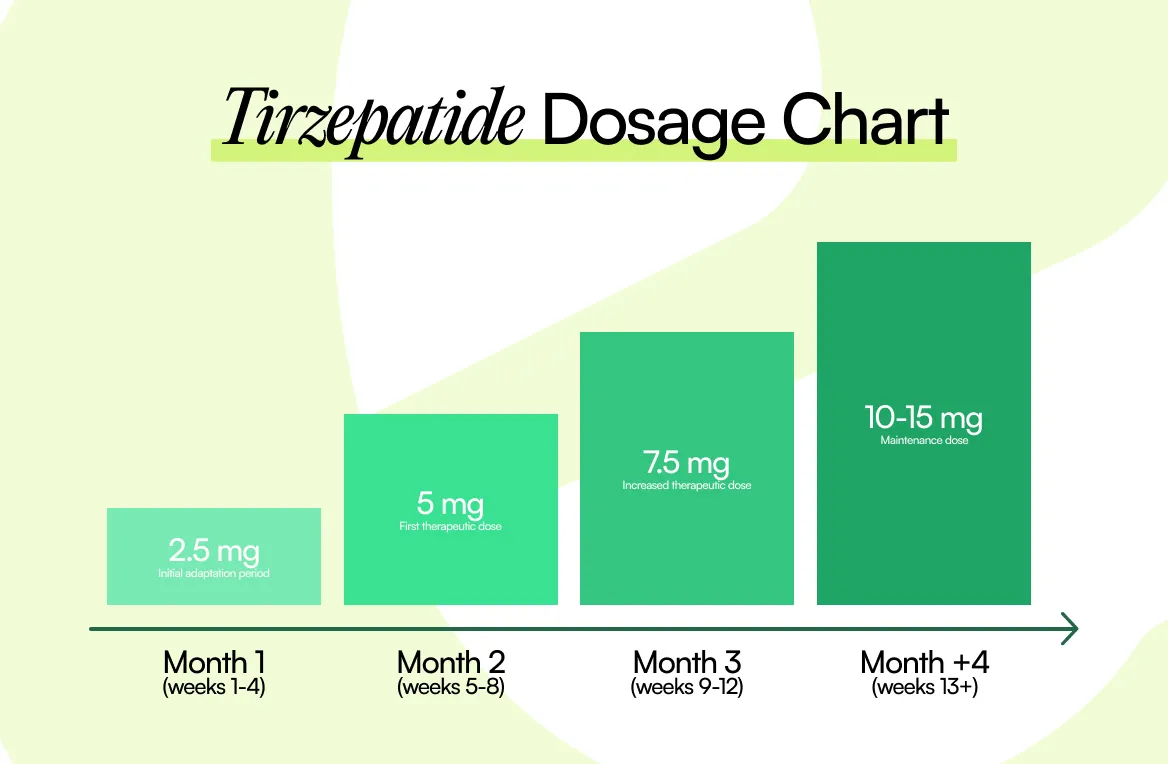
In clinical trials of Zepbound® lasting up to 72 weeks, researchers tracked a wide range of side effects. Below are the most commonly reported reactions, listed by dose compared to placebo.
Gastrointestinal (GI) Side Effects in Zepbound® Clinical Trials
Other Common Side Effects
It’s important to remember that not everyone gets side effects, and most people who do say they’re mild to moderate. Still, knowing what’s common can help you prepare.
When Do Side Effects Start–And Do They Go Away?
Many people notice side effects early, especially as their dose increases. That’s also when GI symptoms tend to be most noticeable, like feeling full sooner, nausea, or constipation.
One reason this can happen is that tirzepatide slows stomach emptying, especially earlier in treatment. For many people, the body adapts over time, but everyone’s timeline is different.
If you’re struggling, it’s worth asking your prescriber whether you can stay on your current dose longer before increasing. Many clinicians already do this when symptoms flare.
{{primary-cta}}
How to Manage Zepbound® Side Effects
Most people can reduce the side effects they experience with a few small changes and a little patience. One reason for GI side effects is that tirzepatide slows gastric (stomach) emptying, especially earlier in treatment or after a dose increase. For many people, symptoms ease as the body adjusts, but everyone’s timeline is different.
Here are a few strategies to help:
If you feel nauseated
- Try eating smaller meals, eat slowly, and stop the moment you feel “comfortably full.”
- Choose bland, lower-fat foods as these are often easier on your stomach. Greasy foods can be harder to tolerate.
- Take small sips of water instead of drinking large amounts at once.
- If your nausea gets worse after a dose increase, ask your provider if you can stay on the current dose longer before increasing it again.
If you have diarrhea
- Hydrate
- Temporarily choose simple foods (like rice, toast, bananas) and gradually add back fiber.
If you get constipated
- Fluids + walking go a long way.
- Add fiber slowly—a sudden jump can worsen bloating.
- If it’s persistent, ask your clinician what OTC options may be appropriate for you.
If you have reflux or heartburn
- Avoid lying down right after eating.
- Try smaller dinners and identify trigger foods (fried, spicy, acidic).
If the injection site is irritated
- Rotate sites as instructed (abdomen, thigh, upper arm).
- Avoid injecting into bruised, tender, or scarred areas.
Serious Side Effects: Warning Signs You Shouldn’t Ignore
Most side effects are annoying, but not dangerous. However, if you notice the following symptoms, it’s important to contact your healthcare provider right away.
Pancreatitis (rare, but important)
Get urgent care for severe, persistent belly pain, sometimes moving to the back, with or without vomiting.
Gallbladder problems (gallstones or gallbladder inflammation)
Call a clinician if you notice right upper abdominal pain, fever, jaundice (yellowing of the skin/eyes), or pale/clay-colored stools.
Kidney injury (often related to dehydration)
If vomiting/diarrhea is intense and you can’t keep fluids down, or you’re peeing much less than normal, contact a clinician promptly.
Severe allergic reaction
Trouble breathing, swelling of the face/throat, or widespread hives/rash = emergency.
Low blood sugar (hypoglycemia)
This is especially a risk if tirzepatide is used with insulin or sulfonylureas. Symptoms include sweating, shakiness, confusion, and a rapid heartbeat. If you have diabetes meds in the mix, your clinician may adjust doses.
Mood changes / suicidal thoughts
Mood or behavior changes should be taken seriously. If you experience suicidal thoughts, seek help quickly.
Thyroid tumor warning (boxed warning)
Contact your clinician if you notice a neck lump/swelling, hoarseness, trouble swallowing, or shortness of breath. Zepbound® is contraindicated in patients with a personal/family history of MTC or MEN2.
{{primary-cta}}
Who Should Not Use Zepbound®?
This isn’t a complete list, but common examples include:
- Personal/family history of MTC or MEN2
- Prior serious allergic reaction to tirzepatide or ingredients
- Severe GI disease (Zepbound® is not recommended in severe gastroparesis)
Your clinician should also review your full health history, especially pancreas, gallbladder, kidney, and eye history, as well as your current meds.
Zepbound® vs Mounjaro® Side Effects: What’s The Difference?
Zepbound® and Mounjaro® both contain tirzepatide, so there’s significant overlap between the two. Especially the GI effects and the boxed warning. Your experience may still differ depending on dose, other meds, and your underlying health.
What Happens If You Stop Tirzepatide?
A common concern is, “If I stop, will I regain the weight?” In studies where people stopped tirzepatide after losing weight, many regained a meaningful amount of weight over the next year. If you’re thinking about stopping because of side effects, cost, or anything else, talk with a clinician about the safest way to stop and what support you can use to maintain progress.

Blog Components



The FDA does not approve compounded medications for safety, quality, or manufacturing. Prescriptions and a medical evaluation are required for certain products. The information provided on this blog is for general informational purposes only. It is not intended as a substitute for professional advice from a qualified healthcare professional and should not be relied upon as personal health advice. The information contained in this blog is not meant to diagnose, treat, cure, or prevent any disease. Readers are advised to consult with a qualified healthcare professional for any medical concerns, including side effects. Use of this blog's information is at your own risk. The blog owner is not responsible for any adverse effects or consequences resulting from the use of any suggestions or information provided in this blog.
Eden is not a medical provider. Eden connects individuals with independent licensed healthcare providers who independently evaluate each patient to determine whether a prescription treatment program is appropriate. All prescriptions are written at the sole discretion of the licensed provider. Medications are filled by state-licensed pharmacies. Please consult a licensed healthcare provider before making any medical decisions.
Frequently asked questions
Many GI side effects are most noticeable during dose increases and may improve over time, but everyone’s experience varies.
Hair loss was reported in trials and is often associated with weight loss. If it happens, ask a clinician about nutrition, protein intake, and the pace of weight change.
Pancreatitis, gallbladder disease, kidney injury (often related to dehydration), severe allergic reactions, hypoglycemia (especially with certain diabetes meds), and the thyroid C-cell tumor boxed warning.
American Diabetes Association. (2024). Standards of care in diabetes—2024. Diabetes Care, 47(Supplement_1), S1–S187. https://doi.org/10.2337/dc24-SINT
Aronne, L. J., et al. (2024). Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity (SURMOUNT-4). JAMA. https://pmc.ncbi.nlm.nih.gov/articles/PMC10714284/
Centers for Disease Control and Prevention. (2023). Adult obesity facts. U.S. Department of Health & Human Services. https://www.cdc.gov/obesity/adult-obesity-facts/
Dandona, P., Dhindsa, S., Ghanim, H., & Saad, F. (2020). Mechanisms underlying the metabolic actions of testosterone in humans: A narrative review. Diabetes, Obesity and Metabolism, 23(1), 18–28. https://doi.org/10.1111/dom.14206
Del Prato, S., Kahn, S. E., Pavo, I., Weerakkody, G. J., Yang, Z., Doupis, J., Ahn, C. W., Mody, R., van der Aart-van der Beek, A., Broedl, U. C., & Frias, J. P. (2021). Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4). New England Journal of Medicine, 385(6), 503–515. https://doi.org/10.1056/NEJMoa2107519
Eli Lilly and Company. (2022). Mounjaro (tirzepatide) injection, for subcutaneous use prescribing information. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf
Eli Lilly and Company. (2023). Zepbound (tirzepatide) injection, for subcutaneous use prescribing information. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217806s000lbl.pdf
Frias, J. P., Davies, M. J., Rosenstock, J., Pérez Manghi, F. C., Fernández Landó, L., Bergman, B. K., Liu, B., Cui, X., & Brown, K. (2021). Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes (SURPASS-2). The Lancet, 398(10295), 143–155. https://doi.org/10.1016/S0140-6736(21)01324-6
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., Stefanski, A., & Wadden, T. A. (2022). Tirzepatide once weekly for the treatment of obesity (SURMOUNT-1). New England Journal of Medicine, 387(3), 205–216. https://doi.org/10.1056/NEJMoa2206038
Ludvik, B., Giorgino, F., Jodar, E., Frias, J. P., Fernández Lando, L., Brown, K., Bray, R., & Rosenstock, J. (2023). Long-term safety and efficacy of tirzepatide in patients with obesity: Results from the SURMOUNT clinical program. The Lancet Diabetes & Endocrinology, 11(3), 210–222. https://doi.org/10.1016/S2213-8587(23)00003-1
National Library of Medicine. (2025, August 15). Tirzepatide injection. MedlinePlus. https://medlineplus.gov/druginfo/meds/a622044.html
Rubino, D. M., Greenway, F. L., Khalid, U., O’Neil, P. M., Rosenstock, J., Sørrig, R., Wadden, T. A., & Wilding, J. P. H. (2022). Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 1 trial extension. Diabetes, Obesity and Metabolism, 24(8), 1553–1564. https://doi.org/10.1111/dom.14725
Wilding, J. P. H., Batterham, R. L., Davies, M., Van Gaal, L. F., Kandler, K., Konakli, K., Lingvay, I., McGowan, B. M., Oral, T. K., Rosenstock, J., Wadden, T. A., Wharton, S., Yokote, K., & Kushner, R. F. (2022). Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obesity and Metabolism, 24(8), 1553–1564. https://doi.org/10.1111/dom.14725
World Obesity Federation. (2023). World obesity atlas 2023. https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023
Thank you!
We'll be in touch.
Thank you!