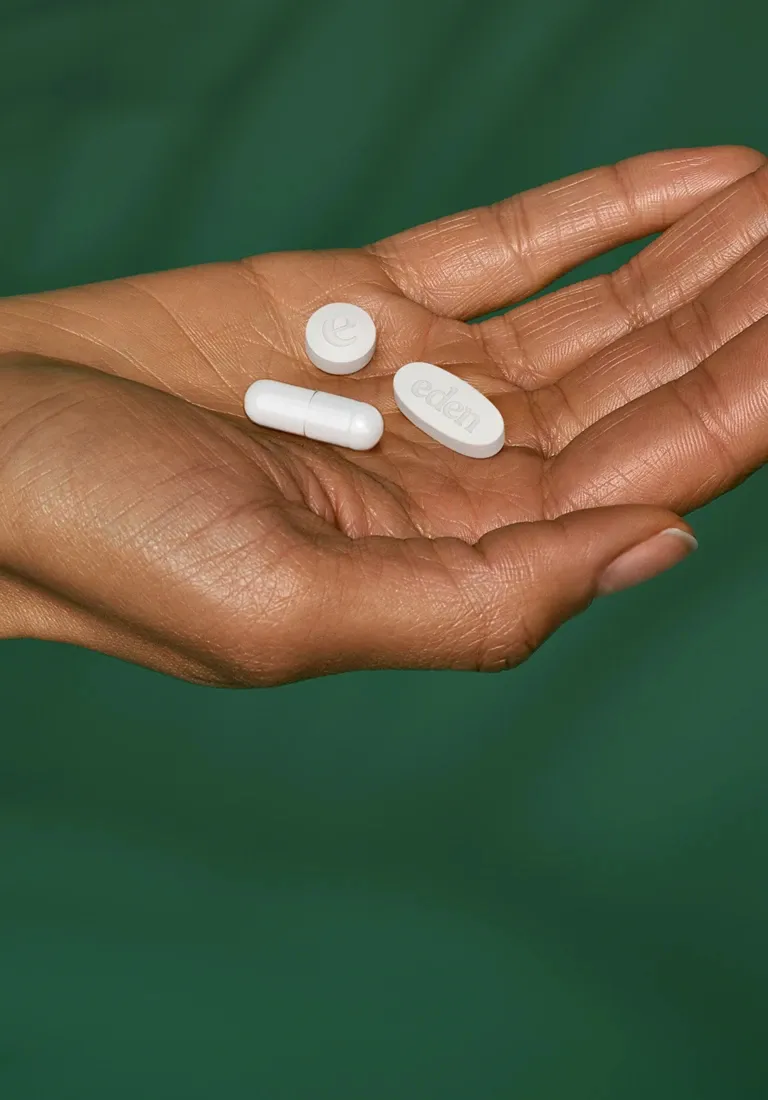
My Custom Weight Loss Kit
Your goals, your plan
Transparent pricing. No surprise fees.
Clinically guided programs that may include designed to support healthy, sustainable weight management as part of a long-term wellness plan.
Each program is developed through a consultation with a licensed provider, who evaluates your health history, goals, and any underlying factors that can influence weight. Based on that assessment, your plan may include one or more prescription treatments such as metformin, bupropion, low-dose naltrexone (LDN), acarbose, or orlistat, alongside supportive nutrients like inositol, acetyl-L-carnitine, and vitamins B6 and B12. These components work through different mechanisms—helping regulate appetite, metabolism, insulin response, and energy utilization.
Yes. Your provider may recommend My Custom Weight Loss Kit as part of a comprehensive plan alongside GLP-1 medications. These treatments are designed to support your body’s natural functions during weight loss, including digestion and energy balance. Your licensed provider will evaluate your health profile to ensure any combination of therapies is safe and appropriate for your individual needs.
Yes. My Custom Weight Loss Kits by Eden may be prescribed on their own, depending on your individual health needs and goals. These plans are developed by licensed providers and may be appropriate for those seeking a non-injectable option for weight management. Your provider will determine if this treatment is a safe and effective fit for you.
Some patients may experience mild side effects, such as nausea or digestive discomfort, especially when starting a new treatment. If you notice any symptoms, you can message your provider directly through the Eden portal. Your care team is here to support you 24/7 and can adjust your plan if needed to help you stay on track safely.
The statements on this page have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
**The FDA does not review or approve any compounded medications for safety or effectiveness.
Feel in control
Oral treatment plans are built by licensed providers around your history, goals, and preferences. No pre-packaged formulas, injections, or gimmicks — just personalized care.
Included with every plan:
- If appropriate: prescription and compounded medications
- Month-by-month dosing instructions and titration protocols
- Secure patient portal and provider access
- Auto-shipped every 90 days

Provider tailored plans for real-life needs
Every plan is personalized to support your unique biology and lifestyle. Providers may include medications for:
Appetite awareness
Blood sugar support
Mood & craving support
Digestive and metabolic routine
Energy & focus
Lab tested medications for quality & potency
.webp)
Lab tested for quality & potency
Clean, simple, and effective
- Cruelty
Free - Eco
Friendly - Paraben
Free - Silicone
Free - Sulphate
Free - Gluten
Free
Hit your health goals safely & affordably in 3 simple steps
Submit your application and meet with a doctor
Step 3
Complete a quick form and meet with a licensed medical provider 100% online. They’ll determine if a personalized treatment plan is right for you.

Get your medication delivered at home
Step 3
If eligible, your custom prescription will be shipped directly to your door, fast and free.

Lose weight with 24/7 support and ongoing care
Step 3
We’ll be with you every step of the way, with regular check-ins and on-demand medical support to keep you on track.

Losing weight is an emotional, physical, and personal challenge that goes beyond just diet and exercise
For many, it's tied to medical conditions that make traditional methods less effective. Prescription medication can help address these underlying issues. Your journey to better health is unique, and Eden is here to support you every step of the way to make real progress toward your health goals.”


Ready for a personalized, non-injectable plan?
Start your online visit and see what your provider recommends for you.
- 1-on-1 guidance from US-based health experts
- No-cost consultations for continuous treatment optimization
- Fast & reliable deliveries with 24/7 support available

Individual results may vary. Testimonials reflect personal experiences and do not guarantee outcomes.
Yes. Your provider may recommend My Custom Weight Loss Kit as part of a comprehensive plan alongside GLP-1 medications. These treatments are designed to support your body’s natural functions during weight loss, including digestion and energy balance. Your licensed provider will evaluate your health profile to ensure any combination of therapies is safe and appropriate for your individual needs.
Yes. My Custom Weight Loss Kits by Eden may be prescribed on their own, depending on your individual health needs and goals. These plans are developed by licensed providers and may be appropriate for those seeking a non-injectable option for weight management. Your provider will determine if this treatment is a safe and effective fit for you.
Some patients may experience mild side effects, such as nausea or digestive discomfort, especially when starting a new treatment. If you notice any symptoms, you can message your provider directly through the Eden portal. Your care team is here to support you 24/7 and can adjust your plan if needed to help you stay on track safely.
Your plan is designed to be flexible. If you’d like to pause, stop, or make changes, you can message your provider anytime through the Eden portal. Your care team will work with you to ensure your treatment continues to align with your goals and current health needs.
Some medications offered through Eden, such as Orlistat, are FDA-approved for weight management. Others, like Metformin or Bupropion, may be prescribed off-label based on your provider’s clinical judgment. Compounded medications, which are custom-prepared for individual patients, are not reviewed or approved by the FDA, but may be recommended when appropriate under medical supervision.
Everyone responds to treatment differently. Some patients may begin to notice changes within a few weeks, while for others, progress may take longer. At Eden, the focus is on steady, sustainable improvement— with your provider monitoring and adjusting your plan to support long-term results based on your individual health needs.
After reviewing your health intake, goals, and medical history, a licensed provider. Your provider will determine if medication is appropriate and which formulations best suit your needs.
No. Eden plans are flexible. Your treatment ships in quarterly supplies, and you can pause or adjust your plan at any time. Your licensed provider will work with you to regularly assess your progress and recommend how long treatment may be appropriate based on your individual health needs.
Yes. Many patients explore additional support as they reduce or transition off GLP-1 therapy. Your licensed provider will review your health history and goals to determine if this treatment is appropriate and can design a plan that complements your current stage of care.
Every Eden plan is built around you. You’ll receive a personalized treatment plan created by a licensed medical provider after a full review of your health profile. If medication is right for you, we’ll craft a custom dosing schedule and ship your treatment straight to your door through a certified, state-licensed pharmacy. You’ll also get unlimited 24/7 messaging with your dedicated care team - so you’re supported every step of the way.
You may also be interested in
Eden connects patients with licensed providers who may prescribe medication through state-licensed pharmacies. Prescription medication only available if prescribed after an online consultation with a healthcare provider. Physicians may prescribe compounded medications as needed to meet patient requirements or drug shortages. The FDA does not review or approve any compounded medications for safety or effectiveness. Results may vary. Plans are offered as a subscription service which you can cancel at any time. Actual product packaging may appear differently than shown.
My Custom Weight Loss Kit
- No hidden fees
- Personalized plans
- On-demand medical support
- Free expedited shipping

Only available if prescribed after an online consultation with a healthcare provider. Benefits outlined are based on third-party studies. Plans are offered as a subscription service which can be canceled at any time. Actual product packaging may appear differently than shown. Physicians may prescribe compounded medications as needed to meet patient requirements. The FDA does not review or approve any compounded medications for safety or effectiveness. The statements on this page have not been evaluated by the FDA. Results may vary. If you notice any side effects while using this treatment, contact your healthcare provider immediately.
Only available if prescribed after an online consultation with a healthcare provider. Benefits outlined are based on third-party studies. Plans are offered as a subscription service which can be canceled at any time. Actual product packaging may appear differently than shown. The FDA does not review or approve any compounded medications for safety or effectiveness. These treatments are individualized and do not guarantee specific results. Provider-determined, off-label use may apply.
- The New England Journal of Medicine: Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin
- American College of Physicians: Annals of Internal Medicine: Long-Term Weight Loss With Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study
- Oxford Academic: Human Reproduction: Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study
- The Lancet: Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
- Obesity: A Research Journal: A Randomized, Phase 3 Trial of Naltrexone SR/Bupropion SR on Weight and Obesity-related Risk Factors (COR-II)
- Obesity: A Research Journal: Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial
- Obesity: A Research Journal: Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women
- American Heart Association: Stroke: Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance
- Journal of the American Medical Association: Weight Control and Risk Factor Reduction in Obese Subjects Treated for 2 Years With Orlistat
- American College of Physicians: Annals of Internal Medicine: Long-term Weight Loss with Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study
- Obesity: A Research Journal: Weight Loss With Naltrexone SR/Bupropion SR Combination Therapy as an adjunct to Behavior Modification: The COR-BMOD Trial
- Mayo Clinic: Pyridoxine (oral route, injection route)
- BMJ Journals: Openheart: Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes
- Mayo Clinic: Acarbose (oral route)
- StatPearls: Orlistat
- National Institutes of Health: Carnitine